
-
Preformulation assessment and formulation development
Quotient Sciences emphasizes the importance of data-driven decisions in early development. Our scientific experts prioritize key API characterization data to recommend the appropriate API form and inform a strategy for preclinical and clinical pharmaceutical development based on the Developability Classification System (DCS).
-
Clinical development and accelerating to proof-of-concept (POC) studies
We offer a full spectrum of manufacturing and supply paradigms, from traditional large batch manufacturing to bright stock distribution and personalized manufacturing. In addition, our ability to produce small batch sizes facilitates conservation of API and dose flexibility. Fundamentally, personalized manufacturing means the product is made on-demand—only when needed and based on patient requirements.
-
Formulation optimization and validation of product performance in humans
We have extensive experience in both traditional formulation development and Translational Pharmaceutics, which combines our drug product and clinical capabilities into a bespoke program that enables formulation decisions based on emerging human data.
-
Process development, scale-up, and clinical manufacturing for Phase II/III
We recognize the need to move rapidly and efficiently through clinical development. So we ensure a seamless transition to larger-scale manufacturing and drug product commercialization by leveraging our expertise to scale up manufacturing processes from Phase I to meet the demands of later clinical trials..
-
Commercial drug product manufacturing and supply
Quotient Sciences configures a robust manufacturing and supply chain to meet every customer’s needs and to maintain seamless continuity throughout the development life cycle. We have experience with accelerated approval pathways to guide development programs through registration and process validation. Our manufacturing facilities support batch sizes ranging from less than 1 kg to over 500 kg for solid oral dosage forms, with plans for a 100k sq ft expansion to be online in 2027. We have the expertise and regulatory approval to manufacture your registration and validation batches for the U.S., U.K., Europe, Japan, China, and other global filing markets. Additionally, our experience extends to supporting 505(b)(2) and all post-approval change filings.
Comprehensive drug product CDMO services
We offer drug product formulation development and drug product manufacturing for all major dosage forms and delivery routes, including:
- Oral solid dosage forms:
- Drug in bottle / blend in bottle
- Drug in capsule / blend in capsule
- Immediate-release tablets, sustained-release tablets, modified-release tablets
- Solubilized formulations, including amorphous spray-dried dispersions (SDD) and hot melt extrusion (HME), micronized and lipidic formulations
- Gels, creams, and ointments
- Oral liquids, solutions, and suspensions
- Inhaled (pulmonary, nasal) dosage forms
- Parenteral dosage forms

Quotient Sciences CMC chemistry manufacturing services

Over 30 years
experience in drug product formulation development and manufacturing.

Scalable batch sizes
ranging small batch sizes to support clinical trial materials, to over 500 kg for solid oral dosage forms in commercial drug product.

Trusted by leading companies
including Fortune 100 large pharmaceutical companies and emerging biotechs.
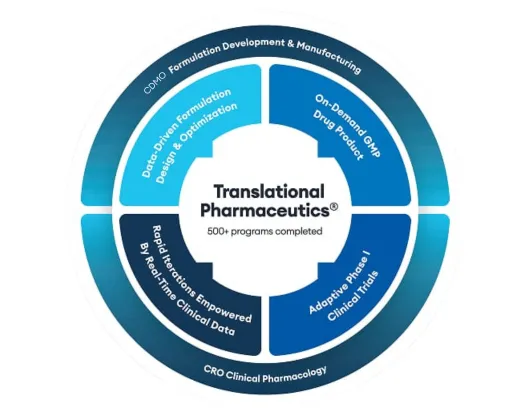
Quotient Sciences Translational Pharmaceutics® platform
Our drug product and clinical pharmacology capabilities combine into a flexible drug development and manufacturing program: Translational Pharmaceutics®.
Quotient Sciences Translational Pharmaceutics® platform helps you accelerate to and through first-in-human trials and optimize formulations coming out of Phase 1 studies, with the ability to release and dose in our clinics in under seven days, adapt to emerging clinical data in real time, and more.
Contact us to learn more about the benefits of integrated Phase 1 clinical studies and drug product services with Translational Pharmaceutics®.
Quotient Sciences is a global accelerator of drug development, with services spanning contract research, development, and manufacturing that make us more than just a traditional CRO or a CDMO.
Our CRDMO offering includes:
More insight, direct from our customers:
"Quotient Sciences has worked with us on several pediatric projects and has been very effective in translating our product concepts into successful prototype formulations."
-Testimonial from a UK-based pharmaceutical company specializing medicines for children
"Quotient Sciences has reduced our time-to-clinic and time-to-market. They understand the issues in drug development and have taken us from early stage to commercial in the most efficient way."
-Testimonial from a former specialty pharmaceutical company
"Quotient Sciences’ expertise in improving the taste, smell, and texture of oral formulations makes them the ideal partner for our pediatric clinical programs."
-Testimonial from a global pharmaceutical company developing therapies for people with endocrine diseases
Small biotech
"Our small biotech company worked with Quotient Sciences on developing a complex formulation and validating the different iterations of the formulation in PK clinical studies using the Translational Pharmaceutics® approach. The project was successful, and we were generally impressed by the skillsets, professionalism, and courtesy of Quotient Sciences' scientists and other subject experts. We have also chosen to work with Quotient Sciences on two subsequent projects."
Biotech with infectious disease focus, acquired by a Fortune 50 pharma company
"Due to the low density and poor flow characteristics of our drug substance, we experienced significant blend and content uniformity issues. By transferring our program to Quotient Sciences, the development team was able to quickly produce demo batches through multiple blending steps and roller compaction using the Gerteis Mini-Pactor. The increase in drug load from 25% to 40% was a significant improvement as we prepared for future Phase II/III trials and this ultimately benefits the patients by reducing the pill burden. We have successfully manufactured several GMP batches of tablets at various doses using a common blend. Quotient Sciences is an outstanding drug development partner with a no-nonsense approach, excellent communication, speed, and robust, easy-to-review documentation, from analytical methods to master batch records."
Former small biotech focused on oncology
"Quotient Sciences is an honest and straight-forward company with a very progressive attitude... What makes them unique is the way in which the pharmaceutical products are made on site and transferred, without leaving the building, to the clinical unit and a good, large and reliable pool of healthy volunteers."
Clinical-stage pharmaceutical company, focused on endocrine therapies
"There is an art to the development of taste-masked oral products for pediatric indications. Quotient Sciences’ expertise in improving the taste, smell, and texture of oral formulations makes them the ideal partner for our pediatric clinical programs."
Your partner to bring new treatments to market. Fast.
From the next generation of emerging biotechs, to Fortune 100 pharmaceutical companies, we have worked with countless companies across the pharmaceutical industry over the last 30 years. Speak with one of our experts to learn more about our capabilities and how we can help you reach your program's milestones.






