
-
Over three decades of proven pharmacology experience
Our experience spans more than 30 years and more than 1,300 Phase I studies delivered at our clinical units in Miami (US) and Nottingham (UK). Our programs are led by experienced project managers alongside skilled scientists and industry-leading Phase I physicians numbering over 15 across both sites. Our team has expertise in the full suite of healthy volunteer studies, including first-in-human (FIH), drug-drug-interaction (DDI), food effect (FE), bioequivalence (BE), thorough QT (TQT) and ADME. You can feel confident that your molecule is in safe hands.
-
Industry-leading clinical project management
Our project management team has over 200 years of collective experience. Each manager leads a matrix team that integrates functions, sites, and stages of development. And they are all committed to forming strong partnerships and true collaboration—by putting you at the heart of how we deliver every project. It’s why over 90% of our customers say they plan to work with us again in the future.
-
High-quality regulatory submissions and fast approvals
Accelerate your study start-up at our clinical units in Miami (US) and Nottingham (UK) with industry-leading IRB and REC/MHRA approval timelines. Our highly experienced regulatory specialists and scientists prepare, submit, and manage your submission. In-depth understanding of the regulatory environment, and strong collaborative relationships with key stakeholders result in a high-quality submissions and fast approvals.
-
Rapid volunteer recruitment and outstanding retention
Our robust databases comprise more than 31,500 active healthy volunteers, and our dedicated recruitment and screening teams are focused on meeting the needs of your study.
We have a volunteer-centric approach, regularly engaging our volunteers to help us maintain our outstanding metrics for delivery. We consider our volunteers to be partners in our clinical trials—their safety, comfort and feedback is important to us. And our statistics prove our success: 99% of our studies start on time; 98% enroll with full cohorts; and 99% of our subjects are retained throughout. -
Seamless delivery of complex protocols from design to reporting
We manage every aspect of end-to-end clinical pharmacology services across healthy volunteer study programs—including data management, analysis and interpretation, all the way through generation of the clinical study report.
Our expert scientific and medical teams advise on your optimal design by leveraging their extensive experience in complex protocols, including multi-part, flexible, and umbrella designs. This approach ensures seamless execution and delivery of high-quality results tailored to your needs, all under a single contract and led by a single project manager.
Looking for a clinical pharmacology partner?
We are focused on delivering the insight you need by offering end-to-end clinical pharmacology services across all Phase I study programs and managing every aspect from expert design to comprehensive data analysis and reporting.
- Trial Design
- Project management
- Medical Writing (synopsis, protocol, ICF)
- Regulatory submissions & management
- Volunteer recruitment & screening
- Pharmacy & drug product preparation
- Clinical conduct
- Data management
- Pharmacokinetics

Clinical Pharmacology in Drug Development

99% of our clinical pharmacology studies
start on time, 98% of the clinical studies enroll with full cohorts, and 99% of our subjects are retained throughout.

More than 1,300 Phase 1 studies completed
over the last 30 years, delivered by our expert teams at our Phase 1 clinical units in Miami (US) and Nottingham (UK).

Active database of 31,500+ volunteers
ready to participate in Phase 1 studies, supported by dedicated recruitment and screening teams focused on your study.
Clinical Pharmacology Services and Study Types
We deliver comprehensive clinical pharmacology services and the full suite of healthy volunteer studies from exploratory to pivotal with the highest quality service and speed. As Phase I specialists, we're skilled in the delivery of many different endpoints.
Clinical Services
- Trial Design
- Project management
- Medical Writing (synopsis, protocol, ICF)
- Regulatory submissions & management
- Volunteer recruitment & screening
- Pharmacy & drug product preparation
- Clinical conduct
- Data management
- Pharmacokinetics
- Statistics
- Clinical & statistical Programming
- Vendor management & quality oversight
Have a question about a service?
Clinical Study Types
- First in human (FIH), including SAD / MAD
- Bioavailability & pharmacokinetics
- Bioequivalence (BE)
- Biosimilars
- Pharmacodynamic & biomarker
- Food effect (FE)
- Drug-drug-interaction (DDI)
- Cardiac monitoring & Thorough QT (TQT)
- Human ADME
- Microdose & microtracer
- Ethnobridging
Looking to start your clinical study?
Endpoints
- Cardiac telemetry
- Holter monitoring
- Laboratory biomarkers, including PBMCs
- CSF sampling
- Taste assessments
- Sleep and EEG recording
- Scales and ratings (e.g. visual analogue scales, diaries etc)
- Ophthalmology measures
- Ultrasound
Have a specific question about endpoints?
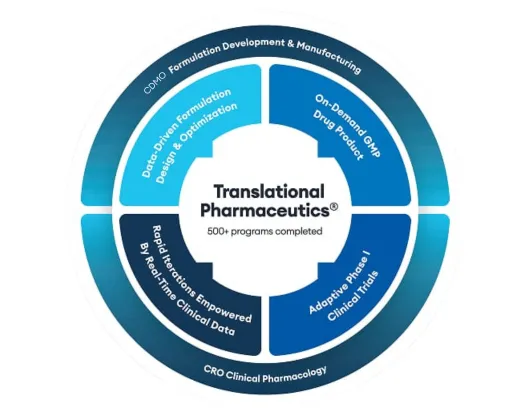
Quotient Sciences Translational Pharmaceutics® platform
Our drug product and clinical pharmacology capabilities combine into a flexible drug development and manufacturing program: Translational Pharmaceutics®.
Quotient Sciences Translational Pharmaceutics® platform helps you accelerate to and through first-in-human trials and optimize formulations coming out of Phase 1 studies, with the ability to release and dose in our clinics in under seven days, adapt to emerging clinical data in real time, and more.
Contact us to learn more about the benefits of integrated Phase 1 clinical studies and drug product services with Translational Pharmaceutics®.
Quotient Sciences is a global accelerator of drug development, with services spanning contract research, development, and manufacturing that make us more than just a traditional CRO or a CDMO.
Our CRDMO offering includes:
More insight, direct from our customers:
"Quotient Sciences has worked with us on several pediatric projects and has been very effective in translating our product concepts into successful prototype formulations."
-Testimonial from a UK-based pharmaceutical company specializing medicines for children
"Quotient Sciences has reduced our time-to-clinic and time-to-market. They understand the issues in drug development and have taken us from early stage to commercial in the most efficient way."
-Testimonial from a former specialty pharmaceutical company
"Quotient Sciences’ expertise in improving the taste, smell, and texture of oral formulations makes them the ideal partner for our pediatric clinical programs."
-Testimonial from a global pharmaceutical company developing therapies for people with endocrine diseases
"Our small biotech company worked with Quotient Sciences on developing a complex formulation and validating the different iterations of the formulation in PK clinical studies using the Translational Pharmaceutics® approach. The project was successful, and we were generally impressed by the skillsets, professionalism, and courtesy of Quotient Sciences' scientists and other subject experts. We have also chosen to work with Quotient Sciences on two subsequent projects."
"Due to the low density and poor flow characteristics of our drug substance, we experienced significant blend and content uniformity issues. By transferring our program to Quotient Sciences, the development team was able to quickly produce demo batches through multiple blending steps and roller compaction using the Gerteis Mini-Pactor. The increase in drug load from 25% to 40% was a significant improvement as we prepared for future Phase II/III trials and this ultimately benefits the patients by reducing the pill burden. We have successfully manufactured several GMP batches of tablets at various doses using a common blend. Quotient Sciences is an outstanding drug development partner with a no-nonsense approach, excellent communication, speed, and robust, easy-to-review documentation, from analytical methods to master batch records."
"Quotient Sciences is an honest and straight-forward company with a very progressive attitude... What makes them unique is the way in which the pharmaceutical products are made on site and transferred, without leaving the building, to the clinical unit and a good, large and reliable pool of healthy volunteers."
"There is an art to the development of taste-masked oral products for pediatric indications. Quotient Sciences’ expertise in improving the taste, smell, and texture of oral formulations makes them the ideal partner for our pediatric clinical programs."






