
-
Flexible, on demand clinical manufacturing
We use a variety of clinical manufacturing approaches to make only the product you need, flexible on demand. This is real-time adaptive manufacturing.
-
Reduce your clinical trial costs and timelines
Improve your clinical trial cost efficiency by reducingdrug substance and drug product waste.
-
On-demand high potency manufacturing
Leverage our expertise in high-potency handling and manufacturing to make your product on-demand.
What can Quotient Sciences’ clinical manufacturing do for me?
We understand the time and cost pressures you face during early-phase evaluation and work with you to ensure a rapid, seamless path from development to clinical trial supply.
We can help you move rapidly through clinical trial design and development to have built the capability to efficiently scale up drug product manufacturing processes to meet the demands of global clinical and patient supply requirements.

Clinical Manufacturing Expertise

Our expertise enables us
to manufacture, package, and release drug products in an accelerated timeframe.

We can help coordinate
global product supply logistics starting with the development and manufacturing of your drug product, then seamlessly integrating a flexible packaging, labeling, and distribution strategy that's tailored to your clinical trial.

We maximize flexibility
around batch size and timing supply to your selected site in response to emerging clinical data or patient recruitment—without affecting the availability of your drug product.
Clinical Manufacturing Services
Find out more about our GMP clinical manufacturing services that will meet your supply needs for investigational medicinal product (IMP) or investigational new drugs (IND) for global clinical trials.
Phase I, II, III Clinical Manufacturing
We support all aspects of your drug product supply for Phase I, II, and III clinical studies:
- Manufacture of all major drug product dosage forms in our US Food and Drug Administration (FDA)- and UK Medicines and Healthcare products Regulatory Agency (MHRA)-approved facilities
- Packaging, including bottles, blisters, and tubs
- Multi-language label design (including translation)
- Post-study drug return and reconciliation
- Randomization and blinding
- Schedule I–IV controlled substance handling
- Shipment logistics and supply tracking
- Storage and distribution capabilities (ambient, refrigerated, and frozen)
- Statistics
- Supply chain management to the clinical site
Have a question about a service?
Dosage Forms
We offer clinical manufacturing and testing services for all major dosage forms, including:
- Solutions and suspensions
- Drug or blend in bottle
- Drug or blend in capsule
- Immediate-, sustained-, and modified-release tablets
- Solubilized formulations, including amorphous (spray-dried and hot melt extrusion [HME]) dispersions, micronized and lipidic formulations
- Gels, creams, and ointments
Have a question about a service?
Delivery Routes
We have extensive experience of developing and manufacturing drug products intended for all major routes of delivery:
- Oral (solid and liquid forms)
- Topical
- Rectal
- Parenteral
Have a specific question about delivery routes?
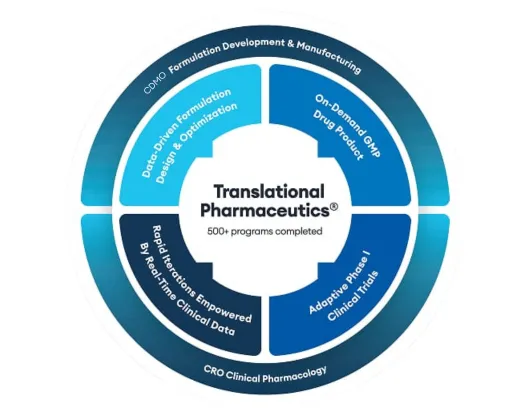
Quotient Sciences Translational Pharmaceutics® platform
Our drug product and clinical pharmacology capabilities combine into a flexible drug development and manufacturing program: Translational Pharmaceutics®.
Quotient Sciences Translational Pharmaceutics® platform helps you accelerate to and through first-in-human trials and optimize formulations coming out of Phase 1 studies, with the ability to release and dose in our clinics in under seven days, adapt to emerging clinical data in real time, and more.
Contact us to learn more about the benefits of integrated Phase 1 clinical studies and drug product services with Translational Pharmaceutics®.
Quotient Sciences is a global accelerator of drug development, with services spanning contract research, development, and manufacturing that make us more than just a traditional CRO or a CDMO.
Our CRDMO offering includes:
More insight, direct from our customers:
"Quotient Sciences has worked with us on several pediatric projects and has been very effective in translating our product concepts into successful prototype formulations."
-Testimonial from a UK-based pharmaceutical company specializing medicines for children
"Quotient Sciences has reduced our time-to-clinic and time-to-market. They understand the issues in drug development and have taken us from early stage to commercial in the most efficient way."
-Testimonial from a former specialty pharmaceutical company
"Quotient Sciences’ expertise in improving the taste, smell, and texture of oral formulations makes them the ideal partner for our pediatric clinical programs."
-Testimonial from a global pharmaceutical company developing therapies for people with endocrine diseases
Small biotech
"Our small biotech company worked with Quotient Sciences on developing a complex formulation and validating the different iterations of the formulation in PK clinical studies using the Translational Pharmaceutics® approach. The project was successful, and we were generally impressed by the skillsets, professionalism, and courtesy of Quotient Sciences' scientists and other subject experts. We have also chosen to work with Quotient Sciences on two subsequent projects."
Biotech with infectious disease focus, acquired by a Fortune 50 pharma company
"Due to the low density and poor flow characteristics of our drug substance, we experienced significant blend and content uniformity issues. By transferring our program to Quotient Sciences, the development team was able to quickly produce demo batches through multiple blending steps and roller compaction using the Gerteis Mini-Pactor. The increase in drug load from 25% to 40% was a significant improvement as we prepared for future Phase II/III trials and this ultimately benefits the patients by reducing the pill burden. We have successfully manufactured several GMP batches of tablets at various doses using a common blend. Quotient Sciences is an outstanding drug development partner with a no-nonsense approach, excellent communication, speed, and robust, easy-to-review documentation, from analytical methods to master batch records."
Former small biotech focused on oncology
"Quotient Sciences is an honest and straight-forward company with a very progressive attitude... What makes them unique is the way in which the pharmaceutical products are made on site and transferred, without leaving the building, to the clinical unit and a good, large and reliable pool of healthy volunteers."
Clinical-stage pharmaceutical company, focused on endocrine therapies
"There is an art to the development of taste-masked oral products for pediatric indications. Quotient Sciences’ expertise in improving the taste, smell, and texture of oral formulations makes them the ideal partner for our pediatric clinical programs."
Your partner to bring new treatments to market. Fast.
From the next generation of emerging biotechs, to Fortune 100 pharmaceutical companies, we have worked with countless companies across the pharmaceutical industry over the last 30 years. Speak with one of our experts to learn more about our capabilities and how we can help you reach your program's milestones.






